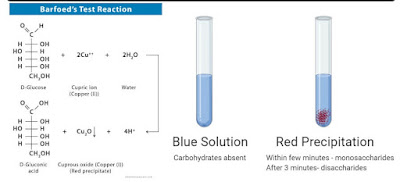
This test is performed to differentiate between mono- and disaccharide reduction. Monosaccharides are more reactive reducing agents than disaccharides and thus react in about 1-2 minutes, while it takes 7-12 minutes for the reducing disaccharides to get hydrolysed and then react in the acidic solution. Therefore it is possible to detect the difference in property reductions.
Reagents
Barfoed's reagent:
66.5 g of Cupric acetate dissolved in approximately 900 mL DW. Add 9 mL of Glacial Acetic Acid and boil. Cool and use DW to cool the volume to 1 L and filter if necessary.
Procedure
- Take 2- solution: Keep the test tubes for only 1-2 min in a boiling water bath.
- Then allow a while for the tubes to cool down.
- Thin red precipitates indicate the presence of a reduction of monosaccharide at the bottom or sides of the tube.
Note:
The boiling should not be prolonged beyond 1-2min, otherwise the disaccharide reduction will respond to this test as well.
This test is not effective in detecting urine sugar reduction due to the presence of chloride ions.
0 komentar
Posting Komentar