The response is based on the fact that H2SO4 concentrated catalyzes the dehydration of sugars to form furfural (from pentoses) or hydroxymethyl furfural (from hexoses). These furfurals then condense to give a purple or violet colored product with sulfonated alpha-naphthol.
Positive reaction is also provided by polysaccharides and glycoproteins. The acid first hydrolyses it into monosaccharides, which are then dehydrated to form furfural or its derivatives, in the case of the carbohydrate being a poly or disaccharide.
Reagents
- Conc.H2SO4
- Molisch's reagent: 5 percent (w/v) of alpha-naphthol in 95% ethanol.
Procedure
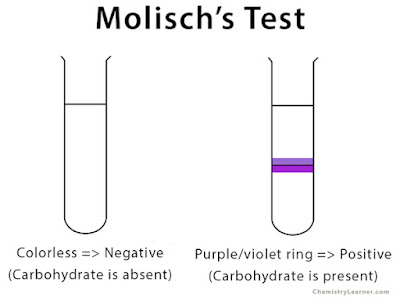
Take 2 mL of the unknown solution and add the contents to the 2-mixture. Incline the tube and pour 1-2 mL of conc.H2SO4 carefully down the side of the tube, so that a layer beneath the aqueous solution
forms the acid. The formation at the junction of two layers of a purple or violet ring or zone indicates the presence of carbohydrates.
Precautions
- The solution of alpha-naphthol is unstable and should be made fresh.
- Conc.H2SO4 should be carefully added along the sides of the test tube, causing the contents of the tube to be minimally disturbed.
Limitations
This test is also performed in addition to carbohydrates, furfurals as such, certain organic acids, aldehydes and ketones. Secondly, due to the charring action of acid, a concentrated sugar solution can give a red colour instead of purple.
0 komentar
Posting Komentar